High volume utilization of conductive adhesives for solar applications began with the increased volume manufacturing of thin-film solar modules. Conductive adhesives are used to attach and make electrical contact between the current carrying ribbons, busbars and back contact sheets of the cells.
By Mark Francis, Rich Wells
.jpg)
.jpg) The photovoltaic market has been dominated by crystalline silicon module manufacturers. For these applications, ribbon stringing is the standard/preferred method for interconnecting the cells. In this process, a tin or tin silver plated copper ribbon is attached to the cell by soldering the ribbon to a high temperature fired silver glass busbar on the top of one cell to the rear contact of the next cell. While this method of interconnection has some technical challenges due to induced stress and residual flux, the challenges are manageable. Some of the advantages of soldering are excellent electrical conductivity, a well-established and relatively fast process, and proven reliability in the field. The photovoltaic market has been dominated by crystalline silicon module manufacturers. For these applications, ribbon stringing is the standard/preferred method for interconnecting the cells. In this process, a tin or tin silver plated copper ribbon is attached to the cell by soldering the ribbon to a high temperature fired silver glass busbar on the top of one cell to the rear contact of the next cell. While this method of interconnection has some technical challenges due to induced stress and residual flux, the challenges are manageable. Some of the advantages of soldering are excellent electrical conductivity, a well-established and relatively fast process, and proven reliability in the field.
Rapid technological advances in module design and composition are expanding the range of applications requiring the use of conductive adhesives to interconnect the cells. These applications are driven by the need to increase efficiency and reduce cost without sacrificing long-term reliability. Any degradation in the bonded interconnect may affect its current carrying capacity. These new applications include thin-film-based modules made from amorphous silicon, cadmium telluride, copper indium gallium selinide or organic PV, which, in most cases, cannot handle the temperatures and stresses associated with high temperature fired pastes and soldering. Even new module designs based on crystalline silicon, including thin silicon and back contact modules will use adhesives to interconnect the cells in most cases.
Just how critical is the conductive adhesive to the long-term reliability of these devices? Solar modules are used on rooftops and grid-connected solar farms where they are exposed to harsh environmental conditions. They need to operate for 20 years with minimal degradation of power output. In each of these applications, the conductive adhesive must have specific properties to provide the long-term reliability demanded by solar panel manufacturers. Solar modules are required to meet the stringent requirements of IEC testing. These tests include thermal cycling from -40¡ÆC to 85¡ÆC, damp heat testing for 1,000 hours at 85¡ÆC/85 percent relative humidity, wind shear and other testing. Does passing the IEC requirements really ensure that these modules will survive for 20 years in the heat of Saudi Arabia, the heat and humidity of Thailand or the harsh winters of Canada? While there are many factors affecting long-term reliability, the conductive stability of electrical interconnects is vital to the long-term reliability of the module. Often, the substrates used for interconnection are oxidizable metals such as tin, tin silver alloys, copper, molybdenum and aluminum. Forming stable bonds and connections to these substrates is challenging at best.
Basics of Adhesive Composition
For this discussion, we will focus on dispensable thermosetting conductive adhesives. Most conductive adhesives used in the industry have the general composition listed below
-Resins--Most common is epoxy.
-Diluents--Reduce the viscosity of the system to enable higher filler loading or better substrate wetting.
-Curing agents--Type depends upon resin chemistry.
-Conductive filler--Most common is silver.
-Other additives--Adhesion promoters, surfactants, conductivity promoters, etc.
These components are mixed/blended together to form a homogeneous mixture under vacuum to remove air and then packaged.
Thermoset Polymers
During application, the adhesive is applied to the substrate by needle dispensing, jetting or printing, the substrates are assembled, and the adhesive is cured by the addition of heat which increases the reactivity of the resins and curing agents. The resins are liquid polymers. A polymer is a chemical chain with repeating chemical units. As the polymers react, the chains become longer and chemical bonds are formed to adjacent chains in a process called cross linking. As the polymer network forms during cure, it gels and then forms a thermoset solid. Cured thermoset polymers are held together by:
-Physical entanglements of the chains
-Secondary chemical bonding forces (polar)?Hydrogen bonds, van der waals forces and london dispersion forces
-Primary bonds?Covalent and/or ionic bonds.
Crosslinks are primary bonds between the chains. Crosslinked polymers are called thermosets.
What is the Glass Transition Temperature (Tg)
In simplistic terms, the Tg is a temperature range in which the thermal/kinetic energy overcomes the secondary bonding forces between the chains, increasing the free volume in the polymer (bond strength decreases for same reason). The polymer goes from a ¡®glasslike¡¯ material below Tg to a ¡®rubbery¡¯ state above Tg. Above the Tg, the Coefficient of Thermal Expansion increases by two to four times, modulus decreases, and there are changes in heat capacity and dielectric constant. When the polymer is cooled, the chains move closer together and the secondary bonds are reformed.
Adhesion
There are many theories of adhesion, a very complex subject. Here, we will keep it very basic and stick to the practical aspects. Adhesives are polar in nature. In order to form bonds to a substrate, the adhesive must ¡®wet¡¯ the surface to be bonded.
We can determine if a surface is bondable by the water break test. Water is polar like the adhesive and will act in a similar way. For bonding, we need to ¡®wet¡¯ the surface of the substrate. In order to do this, the surface free energy must be greater than the surface tension of the liquid. In other words, the liquid must have a stronger attraction to the surface than to itself. We prefer to have an angle less than 300. Rougher surfaces (in most cases) provide more surface area for bonding and increase the mechanical strength.
.jpg)
Most metals, ceramics and polymers containing nitrogen, oxygen, etc. (that have free electrons in non-bonding orbitals, making them polar in nature) have high surface free energy if clean. Materials such as polypropylene, silicone, teflon, etc. have low surface free energy and are generally difficult or impossible to bond to without treatment.
Key Issues in Thin-Film Applications
Thin-film modules (and organic PV) tend to be somewhat temperature-sensitive. Maximum cure temperatures tend to be in the 180¡ÆC range with most customers preferring 150¡ÆC or below to reduce temperature induced thermal stresses. Conductive adhesives can be formulated to cure at these temperatures, but the design engineer must keep in mind that the conductivity of the adhesive is affected by the cure temperature. Most conductive adhesives are filled with silver as the conductor. These silvers are coated with a lubricant to enable dispersion into the base resin system. To enable high conductivity, these lubricants must be dissolved, reacted with or otherwise removed from the silver surface to enable close contact between the silver flakes. The higher the cure temperature, the more efficiently the lubricant is displaced. Many conductive adhesives must be cured above a specific temperature to be conductive at all. It is important to understand the relationship between cure temperature, time and conductivity when choosing an adhesive. Cure time and temperature can be determined using differential scanning calorimetry. The conductivity also should be determined with each specific cure schedule.
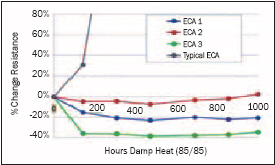
The same tin or tin silver plated copper ribbons used for stringing crystalline silicon cells are used for making interconnects in thin-film devices. Instead of soldering, they are typically bonded with a conductive adhesive in these applications. When bonding tin or tin silver with silver-filled conductive adhesives, galvanic corrosion (also called dissimilar metal corrosion) is a concern that can affect the conductive stability of the interconnect.
.jpg)
The tin acts like an anode (liberates electrons) and the silver as a cathode, creating a galvanic cell that when in contact with water and an ionic species (usually from the adhesive) oxidizes the tin. Under damp heat conditions, this effect is accelerated and the resistivity of the interconnect can increase rapidly. Adhesives can be formulated using specific types of chemistry and formulation techniques to reduce/eliminate this effect. Many of the conductive adhesives being used in photovoltaics today were not formulated for bonding to tin surfaces. If encapsulated well they may pass IEC testing, but if the joint is exposed to moisture, the interconnect may degrade rapidly.
.jpg)
.jpg)
Some thin-film module customers tell us that using an adhesive that maintains conductive stability in damp heat is a critical issue, while others say the modules are sealed and if moisture penetrates their device, it will fail anyway.
In many CIGS applications, the ribbon is bonded to molybdenum on the rear of the cells. During the deposition, a molybdenum oxide would be formed, but during the selenization process a molybdenum selenide is formed. The exact composition of this back surface whether molybdenum selenide, molybdenum oxide, or something in between is not clear to us and certainly varies from manufacturer to manufacturer. How variations in surface composition affect long-term adhesion and conductivity stability also is not as well established. Basically, this becomes a trial and error process in which we see significant differences in damp heat and thermal cycling performance on molybdenum substrates from different customers. While we have developed materials that perform well in damp heat and thermal cycling on the molybdenum substrates that we have tested, we are not clear on the mechanisms and how reliability is affected by varying selenium content/ surface composition.
.jpg)
 In flexible thin-film modules, the rigidity of the adhesive is a key issue. In glass backed rigid modules high glass transition temperature/higher modulus materials with higher adhesive shear strength can be used. Extreme flexibility is required for modules manufactured in reel-to-reel processes. As mentioned before, the glass transition temperature is the center of a temperature range in which an amorphous polymer goes from a rigid, glass-like material to a rubbery material. Above the glass transition temperature, the material is rubbery and stress absorbing. Below, it is ¡®glass-like¡¯ and less stress absorbing. Just how ¡®rubbery¡¯ or ¡®rigid¡¯ the material is depends upon the materials polymer structure. For flexible modules, the conductive adhesive must be flexible enough to distribute the bending stresses across the bond line while maintaining as high a peel and shear strength as possible. In flexible thin-film modules, the rigidity of the adhesive is a key issue. In glass backed rigid modules high glass transition temperature/higher modulus materials with higher adhesive shear strength can be used. Extreme flexibility is required for modules manufactured in reel-to-reel processes. As mentioned before, the glass transition temperature is the center of a temperature range in which an amorphous polymer goes from a rigid, glass-like material to a rubbery material. Above the glass transition temperature, the material is rubbery and stress absorbing. Below, it is ¡®glass-like¡¯ and less stress absorbing. Just how ¡®rubbery¡¯ or ¡®rigid¡¯ the material is depends upon the materials polymer structure. For flexible modules, the conductive adhesive must be flexible enough to distribute the bending stresses across the bond line while maintaining as high a peel and shear strength as possible.
Key Issues for Crystalline Silicon Back Contact Applications
From the perspective of the conductive adhesive supplier, the biggest issue we face is bonding to copper, tin or aluminum substrates, and retaining stable conductivity and adhesion. Most of the back contact applications that we have worked on use silver plated back contact sheets. Due to cost considerations, module designers would like to get away from silver and use copper, tin or aluminum. While silver does not readily oxidize and is a relatively stable substrate to bond to, these other metals readily form oxide layers which make it very difficult to form reliable bonds and conductive joints. We know how to form conductively stable interconnects to tin (see thin film section), but copper and aluminum are different animals.
Differences in the crystalline structure of the base metal copper and copper oxide can form a weak boundary layer. During thermal cycling (stress), copper oxide will delaminate from the base metal. For instance, copper with a face centered cubic crystalline structure and Cu (II) Oxide which has a square planar structure, have poor adhesion between them.

To stabilize the surface Organic Solderability Coating (OSP) treatments are used. The copper surface is passivated with an organic compound that stabilizes the surface, providing a stable surface for bonding and conductive stability. Benzimizazoles and Benzotriazoles are the most common OSP treatments.
In general, the oxide layer is stripped from the copper and the surface is chemically treated before the oxide layer can form. The conductive adhesive must now form stable bonds through the OSP coating to the base metal so the adhesive must be compatible with the OSP chemistry. We have seen some conductive silicones used in the industry (on non-OSP treated substrates), but these OSP chemicals are weak nucleophiles (bases) and may inhibit the cure of the silicone if the silicone cures with a platinum catalyst. OSP treatments are widely used in the semiconductor industry for treating the surfaces of copper lead frames to enable reliable bonds with conductive adhesives between the semiconductor die and the copper surface (die attach).
We do not recommend bonding directly to aluminum with conductive adhesives. Issues with galvanic corrosion and oxidation in general make aluminum a poor choice of substrates, regardless of its cost. We are looking at some ways to potentially stabilize the aluminum surface but, at this time, we are not aware of any proven solution to this technical issue.

Low Cost Conductive Adhesives
It is rare to discuss any issue concerning solar in which cost is not part of the discussion. The price of silver has increased dramatically over the past few years. Additionally, there is a lot of pressure on suppliers of conductive adhesives to reduce the cost of their materials.
Sounds easy! Just replace the silver with less expensive conductive fillers. No problem! There is certainly no shortage of potential replacements including copper, silver plated copper, nickel, nanoparticles, nanotubes, nanowires, graphene, inherently conductive polymers and so on. The suppliers of these materials often make impressive claims, but after 25 years of working on projects to replace silver, there always seems to be some tradeoff in conductivity, processing or long-term stability. With that being said, we are making some headway in cost reduction efforts. At this time, we have materials that are up to 40% less expensive than our standard product line that have reduced silver content. Our current tradeoff is in slightly reduced conductivity.
Whether low-cost conductive adhesives will work in your application depends upon the amount of bulk conductivity required for your specific design. In some designs, the transparent conductive oxide is the limiting factor reducing the effect of higher conductivity in the adhesive. Some designs may see degradation in efficiency from lower conductivity in the adhesive. It is very design specific.
As new technologies emerge in the photovoltaic sector, the use of conductive adhesives is rapidly expanding. Solar module design engineers need to be aware of potential long-term reliability issues concerning their usage. The design engineer must select conductive adhesives capable of withstanding the physical and environmental stresses in photovoltaic systems with minimal degradation over a 20-year product life cycle. To accomplish this, they must fully understand how these materials are applied and cured, their properties, how they bond to surfaces, their interaction with substrates, and the environmental conditions in which they must survive. All of these issues must be considered while keeping costs as low as possible.
Mark Francis received his B.S. in Chemical Engineering from the University of Massachusetts, Lowell and is the Global Business Manager, Solar at Engineered Conductive Materials, LLC. (www.emsadhesives.com)
Rich Wells received his B.A. in Chemistry at Anderson University and earned a M.S. in Polymer Science from the University of Akron and is the Chief Technical Officer at Engineered Conductive Materials, LLC.
For more information, please send your e-mails to pved@infothe.com.
¨Ï2011 www.interpv.net All rights reserved. |


